Endocannabinoids
Endocannabinoids are endogenous {call-out, text:Originating from the body} compounds that bind to the cannabinoid receptors. These compounds are produced by our bodies. Over the years, a variety of compounds with endocannabinoid activity have been isolated or synthesized. Endocannabinoids are fatty compounds that are made from membrane phospholipid precursors, such as omega-6 fatty acids.1 As speculated by researchers in Wageningen, the Netherlands, it has been shown in mice and in humans that one’s diet can indeed influence the amount of endocannabinoids in the blood.2, 3, 4 We will describe two well-studied endogenous cannabinoids: anandamide (AEA)5 and 2-arachidonoylglycerol (2-AG).6 Anandamide (illustration 1) is a fatty acid neurotransmitter {call-out, text:A messenger molecule between two brain or nerve cells}. Anandamide is an endocannabinoid synthesized in a process in which the enzymes N-acyltransferase and N-acylphosphatidylethanolamine-hydrolyzing phospholipase D (NAPE-PLD) form anadamide from membrane phospholipids {call-out, text:Lipids from the outer layer that protects a cell}.7, 8 Its concentration in the body is relatively low and it is transitory, since it is broken down quickly by the enzyme fatty acid amide hydrolase (FAAH).7 It binds to the cannabinoid receptors type 1 (CB1) and type 2 (CB2) where it acts as a partial agonist.9 A number of cannabinoids and phytocannabinoids mimic the effects of anandamide. Anandamide was named after the Sanskrit word ananda, meaning bliss. 2-Arachidonoylglycerol (Figure 2) is synthesized by the enzymes diacylglycerol lipase (DAGL) and phospholipase C-β (PLC) from molecules called arachidonic acid and glycerol.8, 10 2-AG, unlike anandamide, is present at relatively high levels in the central nervous system.8 2-AG is degraded by a process called hydrolysis, which is mainly accomplished by the enzyme monoglyceride lipase (MAGL).10 It acts as a full agonist at CB1 & CB2.8, 11, 12 It has been shown that 2-AG is a major endocannabinoid involved in retrograde signaling.8, 13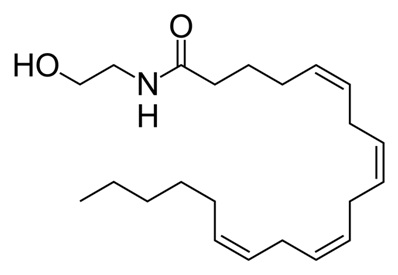 Figure 1: Anandamide (AEA) – an arachidonic acid-containing lipid molecule generated from membrane glycerophospholipids (image taken from Wikimedia).
Figure 1: Anandamide (AEA) – an arachidonic acid-containing lipid molecule generated from membrane glycerophospholipids (image taken from Wikimedia).
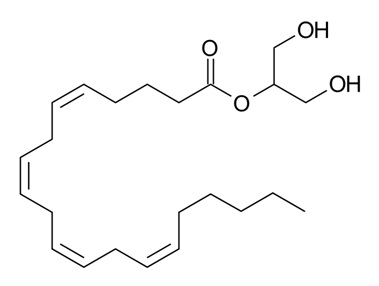 Figure 2: 2-Arachidonoylglycerol (2-AG) – a monoacylglycerol (MAG) molecule containing an esterified arachidonic acid chain at sn-2 position of the glycerol backbone10 (image taken from Wikimedia).
References:
Figure 2: 2-Arachidonoylglycerol (2-AG) – a monoacylglycerol (MAG) molecule containing an esterified arachidonic acid chain at sn-2 position of the glycerol backbone10 (image taken from Wikimedia).
References:- Kreitzer, Anatol C.; Regehr, Wade G. (2002). Retrograde signaling by endocannabinoids. Current Opinion in Neurobiology, 12(3), 324--330.
- Balvers, Michiel G. J.; Verhoeckx, Kitty C. M.; Bijlsma, Sabina; Rubingh, Carina M.; Meijerink, Jocelijn; Wortelboer, Heleen M.; Witkamp, Renger F. (2012). Fish oil and inflammatory status alter the n-3 to n-6 balance of the endocannabinoid and oxylipin metabolomes in mouse plasma and tissues. Metabolomics, 8(6), 1130--1147.
- Wang, Ya; Esser, Diederik; Balvers, Michiel; Chen, Yong-hao; Schutte, Sophie; Vincken, Jean-Paul; Gruppen, Harry; Afman, Lydia A.; Witkamp, Renger F.; Meijerink, Jocelijn (2018). Postprandial effects on endocannabinoid plasma profiles and fat tissue cb1 expression with a high calorie mixed-meal test in obese humans upon a 12 weeks randomised controlled trial with 2 different energy-restricted diets. Annual Symposium of the International Cannabinoid Research Organization (53).
- Witkamp, Renger F.; Balvers, Michiel G. J. (2016). Analysis of Omega-3 Fatty Acid Derived N-Acylethanolamines in Biological Matrices. Methods in molecular biology (Clifton, N.J.), 1412, 27--40.
- Devane, W. A.; Hanus, L.; Breuer, A.; Pertwee, R. G.; Stevenson, L. A.; Griffin, G.; Gibson, D.; Mandelbaum, A.; Etinger, A.; Mechoulam, R. (1992). Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science (New York, N.Y.), 258(5090), 1946--1949.
- Mechoulam, R.; Ben-Shabat, S.; Hanus, L.; Ligumsky, M.; Kaminski, N. E.; Schatz, A. R.; Gopher, A.; Almog, S.; Martin, B. R.; Compton, D. R. (1995). Identification of an endogenous 2-monoglyceride, present in canine gut, that binds to cannabinoid receptors. Biochemical pharmacology, 50(1), 83--90.
- Deutsch, Dale G.; Chin, Suzette A. (1993). Enzymatic synthesis and degradation of anandamide, a cannabinoid receptor agonist. Biochemical Pharmacology, 46(5), 791--796.
- Wang, Jun; Ueda, Natsuo (2009). Biology of endocannabinoid synthesis system. Prostaglandins & Other Lipid Mediators, 89(3-4), 112--119.
- Sugiura, T.; Kobayashi, Y.; Oka, S.; Waku, K. (2002). Biosynthesis and degradation of anandamide and 2-arachidonoylglycerol and their possible physiological significance. Prostaglandins, Leukotrienes and Essential Fatty Acids (PLEFA), 66(2-3), 173--192.
- Ueda, Natsuo; Tsuboi, Kazuhito; Uyama, Toru; Ohnishi, Taira (2010). Biosynthesis and degradation of the endocannabinoid 2-arachidonoylglycerol. BioFactors, 37(1), 1--7.
- Sugiura, T.; Kodaka, T.; Nakane, S.; Miyashita, T.; Kondo, S.; Suhara, Y.; Takayama, H.; Waku, K.; Seki, C.; Baba, N.; Ishima, Y. (1999). Evidence that the cannabinoid CB1 receptor is a 2-arachidonoylglycerol receptor. Structure-activity relationship of 2-arachidonoylglycerol, ether-linked analogues, and related compounds. The Journal of biological chemistry, 274(5), 2794--2801.
- Sugiura, T.; Kishimoto, S.; Oka, S.; Gokoh, M. (2006). Biochemistry, pharmacology and physiology of 2-arachidonoylglycerol, an endogenous cannabinoid receptor ligand. Progress in Lipid Research, 45(5), 405--446.
- Justinová, Zuzana; Yasar, Sevil; Redhi, Godfrey H.; Goldberg, Steven R. (2011). The Endogenous Cannabinoid 2-Arachidonoylglycerol Is Intravenously Self-Administered by Squirrel Monkeys. The Journal of Neuroscience, 31(19), 7043 LP ---- 7048.
_logo.svg)